
- Medical
- Irrigation
- Agricultural & Farming
- Equipment
- General industrial
- Aerospace
- Automotive and defense
- Research & Lab equipments
- Electronics
- Construction
- Communications
- House wares & Consumer Products
- Cosmetics and Personal Care Products
Value Added Secondary Services
In addition to continuously improving our molding capabilities, Hi-Rel Plastics & Molding, Inc. have years of experience in proven advanced molding technologies like
The rising use of plastics in medical devices means that the capability of being sterilized is
rapidly becoming a key selection criterion for any plastic to be used in a medical device. The
objective of sterilization is to prevent the introduction into the body of pathogenic organisms
not normally present. Sterilization can be defined as ‘the removal or destruction of all living
organisms, including resistant forms such as bacterial or fungal spores’.
Bacterial spores are most resistant to destruction, and if the sterilization is effective in
eliminating bacterial spores then it can generally be assumed that all other pathogenic and
non-pathogenic organisms have been destroyed. Disinfection is a lower grade of sterilization and
involves only the destruction of pathogenic organisms in the vegetative (or non-sporing) state;
it does not involve the destruction of spores.
Sterilization is the only acceptable standard for surgical purposes although disinfection may
well be suitable for other purposes. Sterilization Methods Sterilization can be achieved through
a variety of methods and these will be considered individually with particular emphasis on the
applicability of the method to the sterilization of plastics devices. No matter which
sterilization method is used, the objective is to reduce the bioburden (the number of
microorganisms present) to a safe level. [Production in a ‘clean room’ (of any standard) does
not make a device sterile; it simply reduces the initial bioburden and concentration of foreign
particles to make sterilization more effective.]
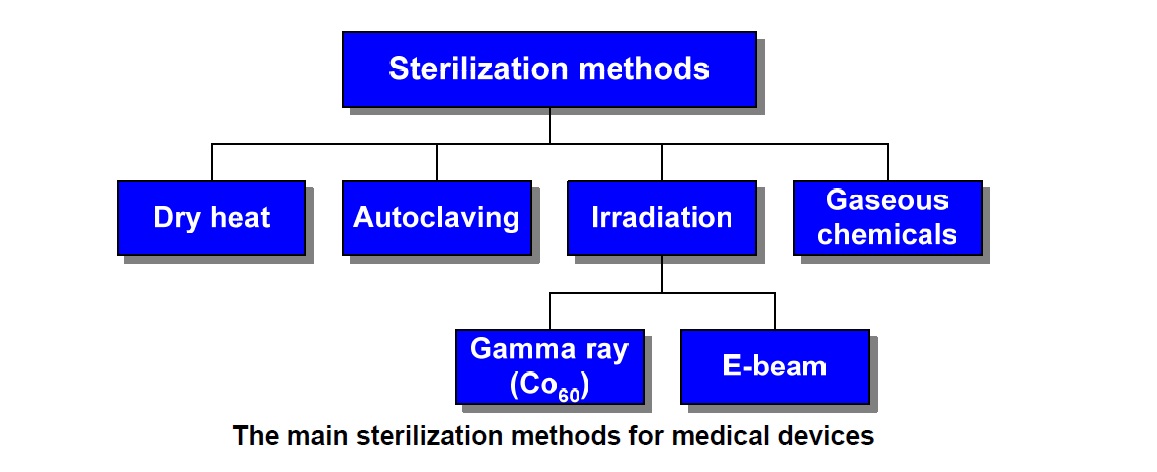
The main sterilization methods for medical devices are:
• Dry Heat
• Autoclaving
• Irradiation
• Gamma Rays
• E-beam
• Gaseous Chemicals (EtO)


